Global LAD Market Overview
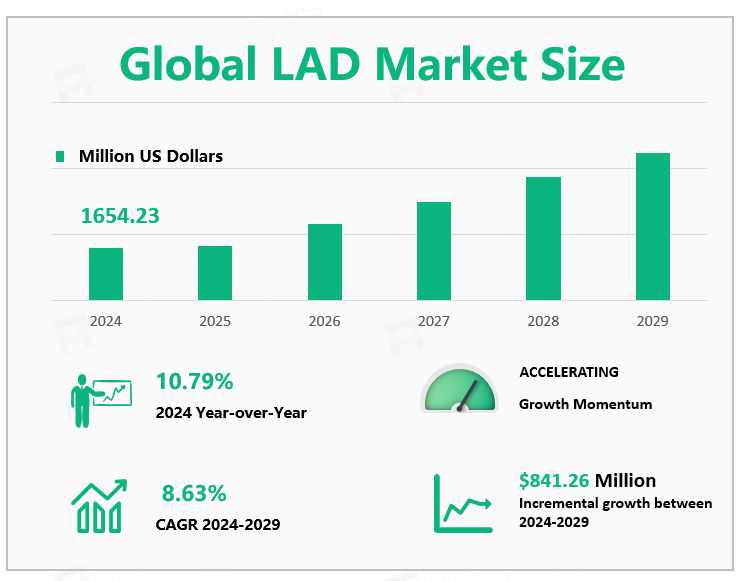
According to Global Market Monitor, the globalLVAD market size is $1654.23 million in 2024 with a CAGR of 8.63% from 2024 to 2029.
A left ventricular assist device (LVAD) is a pump used for patients who have reached end-stage heart failure. Doctors surgically implant the LVAD, a battery-operated, mechanical pump, which then helps the left ventricle (main pumping chamber of the heart) pump blood to the rest of the body. LVADs can be used as Bridge-to-transplant therapy and Destination therapy.
A left ventricular assist device (LVAD) can save the life of a patient with heart failure. These mechanical pumps, implanted in the chest cavity, deliver oxygen-rich blood to various parts of the body when needed to help the patient's heart function properly. For the downstream market, implantation is a necessity if needed, so the impact on downstream demand is not significant.
High Barriers to Market Assessment
The LVAD industry requires a high level of talent and technology, and the LVAD industry involves multiple disciplines, including biology, materials science, electronics, and computer science, in addition to clinical medicine. In addition to clinical medicine, the LVAD industry involves multiple disciplines, including biology, materials science, electronics, computers, etc. The R&D team of an enterprise needs long-term investment and experience to reach a certain level, and new entrants cannot acquire technology in a short period of time.
Market Drivers
Heart failure and stroke is one of the key factors driving the growth of the market. Although heart transplantation (HTx) is the best surgical treatment for end-stage heart failure (HF), this treatment option is becoming increasingly limited. The gap between donor and recipient is widening due to lack of suitable heart donors. LVAD implants are life-saving devices for patients with severe heart failure. They help minimize the symptoms of heart failure, thereby helping patients return to daily activities. Coronary heart disease associated with high blood pressure is a very common cause of chronic heart failure. In addition to this, other heart conditions such as ischemic and dilated cardiomyopathy require significant surgical intervention. Heart transplantation is a long-term successful procedure for all patients who require advanced heart failure-related treatment. However, the waiting time is generally long because of the low supply of organs. Such cases require immediate assistance from an LVAD, which can serve as a bridge to transplantation. Here, the demand for and availability of LVADs plays a crucial role and hence, the increasing burden of heart disease and heart failure is a major force driving the LVAD market globally.
Market Players
Abbott is one of the major players in the market with a 46.25% market share in 2024.
On July 25, Abbott Heart Mate 3 implantable left ventricular assist system was approved by the National Medical Products Administration (NMPA). As the world's first approved "artificial Heart" using full magnetic levitation flow technology, Heart Mate 3 is currently the only left ventricular assist system that has obtained the three major certifications of NMPA, EU CE and FDA. Heart Mate 3 was approved by the FDA to enter the market in 2017 and has the largest clinical trial data to date, showing that it can extend the survival of patients with advanced heart failure by about 5 years (median survival is more than 5 years), providing significant survival benefits to patients.
|
Company Name |
Abbott |
|
Website |
www.abbott.com |
|
Established Time |
1888 |
|
Business Distribution Region |
Worldwide |
|
Business Overview |
Abbott Laboratories discovers, develops, manufactures, and sells a broad and diversified line of health care products and services. The Company's products include pharmaceuticals, nutritional, diagnostics, and vascular products. Abbott markets its products worldwide through affiliates and distributors. |
We provide more professional and intelligent market reports to complement your business decisions.