In vitro diagnostics (IVD) refers to a diagnostics
method that involves testing human samples (such as blood, body fluids,
tissues, etc.) outside the human body to obtain clinical diagnostics
information and determine diseases or body functions. It is a powerful tool for
medical testing, providing comprehensive (such as biochemical information,
immune information, and genetic information) and multi-level (such as
qualitative, semi-quantitative, and quantitative) testing information, becoming
an important source of clinical diagnostics information.
Introduction to In Vitro Diagnostics Classification
|
Biochemical Diagnostics
|
Mainly used for routine testing, such as blood
routine, urine routine, etc. The principle is to measure biochemical
indicators in the body through various biochemical reactions, such as
enzymes, sugars, lipids, protein and non-protein nitrogen, inorganic
elements, etc.
|
|
Immunodiagnostics
|
It covers a wide range of testing items,
including infectious diseases, HIV, viruses, tumors, etc. According to
different technologies, immunodiagnostics can be further subdivided into
methods such as chemiluminescence and time-resolved fluorescence.
|
|
Molecular Diagnostics
|
Narrowly speaking, it mainly refers to nucleic
acid diagnostics, which determines the status of diseases through the
detection of nucleic acids.
|
|
Microbial Diagnostics
|
Determine the status of a disease by detecting
microorganisms.
|
|
Blood Diagnostics
|
Judging the condition of a disease through blood
testing.
|
|
Point-of-Care Testing (POCT)
|
This is a diagnostics method that can be
performed on-site quickly yield results, and can be operated by non-professionals.
|
Overview of Market Development and Analysis of Market Development in Major Regions
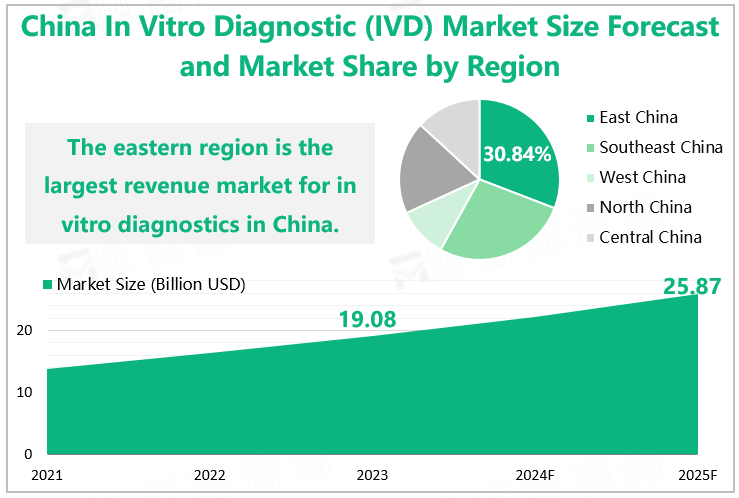
Driven by multiple factors such as the progress of medical technology, the acceleration of population aging, the increase of chronic disease incidence rate, and the improvement of the government's emphasis on medical health, China's IVD market has developed rapidly in recent years. According to our research data, the size of China IVD market reached $19.08 billion in 2023, among which the segmented market size in east China was $5.88 billion, with a market share of 30.84%, making it the largest revenue market for IVD in China; The Southeast region ranked second with market size of $5.19 billion and a share of 27.21%; The northern region ranked third with a share of 18.72%.
In the future, with the advancement of technology and the growth of medical demand, the market is expected to continue to maintain a good development trend. It is expected that by 2025, the size of China's in vitro diagnostics market will increase to $25.87 billion.
China In Vitro Diagnostics (IVD) Market Size Forecast and Market Share by Region
Source: www.globalmarketmonitor.com
Market Development Trend Prediction
Technological innovation drives market development: In vitro diagnostics technologies continue to innovate, such as gene sequencing, immune diagnostics, molecular diagnostics, and other new technologies continue to emerge, which will drive market development. These new technologies will provide more accurate and rapid diagnostics results for clinical practice, improving the accuracy and efficiency of diagnostics.
Policy support helps market development: The Chinese government has attached great importance to the medical and health sector and has introduced a series of policies to support the development of the in vitro diagnostics market. For example, policies such as encouraging innovation, strengthening regulation, and improving medical security will provide a favorable development environment for the in vitro diagnostics market.
The competitive landscape is becoming increasingly fierce: With the development of the market, more and more enterprises are entering the field of in vitro diagnostics, and the competition will become even more intense. Enterprises need to continuously improve product quality, reduce costs, and strengthen brand building to enhance competitiveness.
Diversified market demand: With the increase in medical demand, the demand for in vitro diagnostics will also show a diversified trend. Different diseases and patient groups require different diagnostics methods and reagents, which will drive in vitro diagnostics enterprises to continuously develop new products and services to meet market demand.