Global Eosinophilic Esophagitis Drug Market Overview
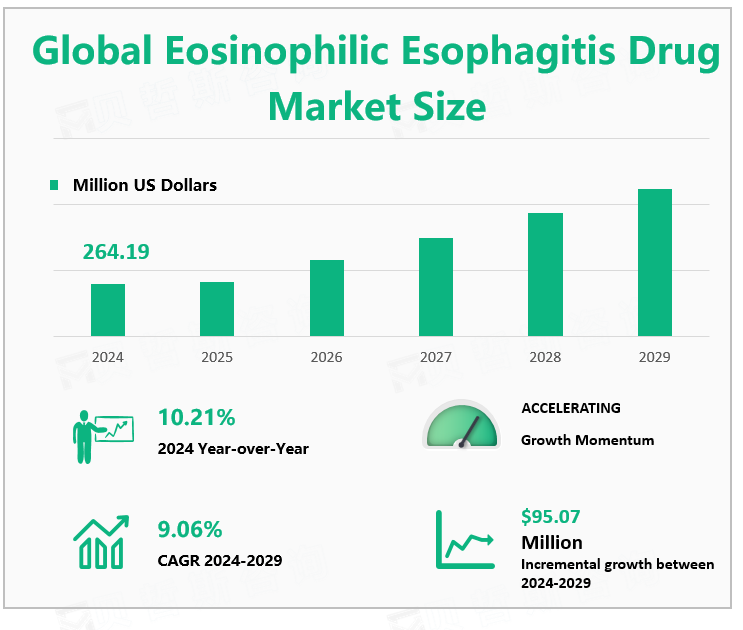
According to Global Market Monitor, the global eosinophilic esophagitis drug market size is $264.19 million in 2024 and is expected to grow to $359.26 million by 2029.
Eosinophilic esophagitis, also known as allergic oesophagitis, is an allergic inflammatory condition of the esophagus that involves eosinophils, a type of white blood cell. Symptoms are swallowing difficulty, food impaction, vomiting, and heartburn. Eosinophilic esophagitis was first described in children but also occurs in adults. The condition is not well understood, but food allergy may play a significant role. The treatment may consist of removal of known or suspected triggers and medication to suppress the immune response.
Strict regulatory policies in the pharmaceutical industry
The production of medicines must maintain high standards to ensure the strength of the active ingredients and the quality and purity of the final product. These standards ensure that safe and effective products are provided for patients. Therefore, most governments in the world have implemented regulations on pharmaceutical companies, and each country has strict regulations on the production and marketing of drugs. These regulations often extend the process of bringing new drugs to the market. However, because EoE is a rare chronic esophageal inflammatory disease, pharmaceutical companies can apply for orphan drug qualification. This is because the government encourages pharmaceutical companies to develop treatments for rare diseases. These incentives may include faster approval times and financial assistance.
Market News
Nov. 06, 2024 -- Regeneron Pharmaceuticals, Inc. and Sanofi announced that the European Commission (EC) has approved Dupixent (dupilumab) to treat eosinophilic esophagitis (EoE) in children as young as 1 year of age. Specifically, the approval covers children aged 1 to 11 years who weigh at least 15 kg and who are inadequately controlled by, intolerant to, or who are not candidates for conventional medicinal therapy. This expands the initial approval in the European Union (EU) for EoE in adults and adolescents and makes Dupixent the first and only medicine indicated to treat these young patients. Dupixent is also approved in this young age group in the U.S. and Canada.
February 12, 2024 – Takeda announced that the U.S. Food and Drug Administration (FDA) has approved EOHILIA (budesonide oral suspension), the first and only FDA-approved oral therapy for people 11 years and older with eosinophilic esophagitis (EoE).
|
Major Players |
|
AstraZeneca(AstraZeneca holds a share of 32.67% in 2024.) |
|
GSK |
|
Bayer |
|
Teva |
|
Dr. Falk Pharma |
We provide more professional and intelligent market reports to complement your business decisions.